Measuring CTS
A scientific approach to disease involves measuring things, and although it is undoubtedly true that there are some things which cannot be measured it is surprising how much can be measured when a little ingenuity is applied. This page is probably rather dry for the average reader but most of the information in the rest of the site is derived from making measurements of one kind or another on thousands of patients with (and without) CTS. Here you will find a catalogue of the tools which we and others have used to study CTS and some notes on their advantages and shortcomings.
There are many ways of measuring CTS, this page discusses:
Condition specific severity scales for CTS
Region specific disease severity scales
Physiological measurement
Variously known as - electrophysiology, neurophysiology, EDX, NCS, EMG
Carpal tunnel syndrome is, first and foremost a disorder of nerve, manifested by symptoms which either indicate irritation of nerve (pain, tingling etc) or loss of nerve function (numbness, weakness). Since the 1930s it has been possible to measure the speed of signal conduction along a large nerve and the size of the electrical signal generated by signals travelling simultaneously in many nerve fibres together – a measurement which serves as a proxy for the number of cells being measured. These laboratory measurements (See HERE for a full description) are fairly obviously numerical in nature and can be analysed mathematically and used as an indicator of the severity of nerve injury but they do not have direct meaning for the patient. For ease of use in CTS, multiple different measures of median nerve function can be combined to form the Canterbury Severity Scale for CTS.
Anatomical measurement
With the advent of imaging methods which can show sufficient detail of soft tissues, CT scanning, MRI scanning and ultrasound imaging, it has become possible to measure the dimensions of the carpal tunnel and it’s contents in a live patient. It has also become obvious that patients with CTS have changes in the dimensions of the nerve which alter as the disorder progresses, or responds to treatment. More sophisticated imaging is becoming available which can measure blood flow in tissue or the stiffness of tissue or other physical properties. All of these methods, like the physiological measurements, produce numbers which can be handled mathematically in studying CTS but, like the physiological measures, the numbers derived from imaging do not have immediate relevance to the patient – until we look at your nerve with the ultrasound scanner and measure it you neither know nor care whether your median nerve measures 8 mmsq across or 10 mmsq.
Subjective severity - what does CTS feel like to the patient?
How you experience your symptoms is, of course, personal to you. However, through our remarkable human facility with language we are able to tell each other something about our symptoms and by standardising the ways in which we describe symptoms and their intensity it is possible to obtain descriptions of symptoms which can be expressed as numbers and manipulated mathematically. The immediate question which occurs to most patients on meeting the assessment methods described below is – “How do I know what is supposed to be ‘mild’ or ‘severe’ pain?” - or whatever other symptom is the subject of enquiry. There are two factors which allow us to make use of these things despite the uncertainty around individual interpretation. Firstly, when these tools are used with many patients as a group, the variations in interpretation average out. Secondly, many of these are used to compare the answers given by the same patient at different times, for example before and after treatment, and in those circumstances we can assume that your interpretation of the language will remain relatively constant.
These tools are therefore used in several ways:
a) As part of the initial assessment
b) After treatment as a standardized way of recording symptoms
c) As a comparison over time – typically before and after treatment
Uses b) and c) are termed Patient Reported Outcome Measures (PROMs)
A wide variety of different PROMs have been used in CTS and which is the most appropriate to use depends greatly on what you hope to learn. They vary from measures of a single symptom to attempts to create an assessment of overall quality of life. They are discussed here working from the most to the least specific.
Single symptom measures
A surprising number of studies of CTS have used the patient’s rating of the severity of a single symptom as an outcome measure. The patient may be asked to give a number on a scale, 0-10 for example indicating how severe a symptom is, given the ‘anchor’ points of 0 being none and 10 being the worst they can imagine. Alternatively the patient may be asked to make a mark on a line representing the range of severity from none to the worst imaginable – a so called ‘visual analog scale’ and this can be converted to a number for analysis by simply measuring the distance along the line to the mark. These simple measures have their place when you really want to know about the impact of a treatment on a specific symptom but they have severe limitations as overall measures of the outcome of a treatment. Visual analog scales for pain in particular have been a popular outcome measure in many trials of unconventional treatments for CTS but a significant number of patients with CTS report no pain at all, either before or after treatment. In order to capture the full range of different symptoms reported by CTS patients a better approach is therefore to combine this sort of measure for several different symptoms and add them together to obtain a summary score. If we are especially interested in measuring the patient experience of CTS we can choose a combination of simple scales which ask about symptoms which we know to be common in CTS patients…
CTS specific tools
SSS/FSS - The most widely used of these is a questionnaire published by the Boston group (Levine 1993). This has been shown to be a good indicator of the subjective severity of CTS symptoms and to be responsive to changes as a result of treatment. It is this questionnaire which is incorporated into this website as a part of our larger symptom survey. Thus, if you complete the CTS questionnaire on this site you will not only get an indicator of the probability that you have CTS but also the subjective severity scores derived from Dr Levine's questionnaires. This severity questionnaire does not have a universally accepted name but is variously referred to as the 'Boston' or 'Levine' questionnaire, the CTS severity instrument or as the CTS SSS and FSS - SSS standing for symptom severity scale and FSS for functional status scale. The SSS and FSS are two subscale scores, the SSS indicating how bad the symptoms feel to the patient and the FSS indicating how much interference the symptoms cause with activities of daily living. Each score can range from 1 (no symptoms) to 5 (the worst symptoms).
The original publication was a little vague about exactly how the questionnaire should be answered by patients who had symptoms of differing severity in their right and left hands and various approaches to this have been adopted by other investigators who have used it. So far as I am aware the Boston group themselves now ask patients to answer the SSS questions separately for each hand but the FSS questions just once for both hands together. The approach adopted here is to ask for a complete set of answers for each hand - further details are shown on the relevant questionnaire pages. When you have completed the entire symptom questionnaire on this site once, you can complete the SSS and FSS repeatedly without doing the rest of the questionnaire in order to track changes in your symptoms over time.
Many studies have reported the change in SSS/FSS with different interventions for CTS generally finding a change of about -1.5 in SSS and -0.8 in FSS with surgery. For injection the SSS change is generally about -1.0 and the FSS change about -0.5. However it should be noted that the SSS and FSS have a ‘floor effect’. Scores cannot go below 1 so if one group of patients (having injection for example), starts out with an average score of 2 and a second group (having surgery) starts out with a score of 3, and both groups end up with a score of 1 the CHANGE in score for the surgery group will be bigger than that for the injection group, even if both groups are completely asymptomatic after treatment.
Dr Atroshi has just published a revised version of the Levine/Boston instrument designed to tackle some of the oddities and redundancies in the original questionnaire (Atroshi 2011), but it remains to be seen whether this will be widely adopted. For the present we are continuing to use the original version in the interests of compatibility with all our existing data.
One author (Storey 2009) has suggested ranges of the SSS and FSS which can be equated to ordinary English terms for severity as follows but expressed in terms of total scores (simply adding together the 1-5 scores on each question in each subscale) rather than the usual average score for the subscale:
SSS 11 = Asymptomatic
SSS 12 to 22 = Mild
SSS 23-33 = Moderate
SSS 24-44 = Severe
SSS 45-55 = Very Severe
These scores can be converted into the values used on this site by dividing by 11
FSS 8 = Asymptomatic
FSS 9-16 = Mild
FSS 17-24 = Moderate
FSS 25-32 = Severe
FSS 33-40 = Very Severe
For the FSS you need to divide these figures by 8 to arrive at the values used on this site. Storey suggested that patients with SSS < 23 might legitimately be considered to have their symptoms under control and that this value mihght be used as a threshold for deciding when further treatment is needed. The justification for this suggestion is unclear. In data from the Canterbury CTS clinic, patients who choose not to pursue any further treatment at first presentation have an average SSS of 2.39 (Standard deviation 0.83) - which equates to a sum score of 26 using Storey's way of expressing these scores. For comparison patients opting for injection have an average score of 2.88 (SD 0.82, 32 as a sum score) and those opting for surgery 3.17 (SD 0.76, 35 as a sum score). This suggests to me that the threshold score at which people decide that 'something must be done' is probably a little higher than suggested by Storey - perhaps about 29 (or 2.63 expressed as an average) rather than 23.
GSS -One other CTS specific tool has been used in several studies – the Global Symptom Score (GSS) (Herskovitz 1995). This is a little simpler than the SSS/FSS. The patient is asked to rate each of 5 symptoms on a 0-10 scale – Pain, Numbness, Paraesthesia(tingling), Weakness/Clumsiness, and Nocturnal waking. The 5 scores are then added together to give a total measure of symptom severity which can range from 0 to 50. In a study using this to compare surgery and injection for CTS (Hui 2005) the mean improvement with injection was 8.7 (SD13) vs 24.2 (SD11) in the surgery group.
These instruments are well suited to comparing different treatments for CTS but if a tool is needed which can be used to assess patients with a variety of different disorders then focusing only on symptoms which are commonly found in CTS may fail to take account of other symptoms which may be important to patients. You may wish to have a more generally applicable instrument for convenience of administration, for example you may wish to give the same outcome assessment to every patient attending a hand clinic, regardless of whether they are attending for CTS or for an arthritic thumb. More generic tools are also needed in health economics when decisions have to be made on the relative cost effectiveness of treatments for different diseases. There are several examples of questionnaires designed for use in any upper limb problem:
Hand/arm specific tools
DASH/QuickDASH – the ‘Disabilities of the Arm, Shoulder and Hand’ questionnaire (Hudak 1996). Like several of the more generic tools this exists in a ‘full’ form (78 questions in the original version + 5 ‘optional’ items) and a shortened ‘quick’ form designed for easier completion by patients (Beaton 2005) – 11 questions. QuickDASH scores in a recent Japanese study improved from 31.2 (SD=18.6) to 24.4 (SD = 19.3) after carpal tunnel surgery (Iwatsuki 2014)
Michigan Hand Outcome Questionnaire (Chung 1998) – a very comprehensive (4 pages long) covering 6 domains of upper limb function:
1) Overall
2) Activities of daily living
3) Work performance
4) Pain
5) Aesthetics
6) Satisfaction with hand function
These instruments are very good at providing numerical measures of upper limb health. They are often used to evaluate change in symptoms with time or treatment and in groups of patients they are capable of detecting very small changes in average scores over time. For example the average change in SSS 3 months after carpal tunnel surgery was -1.4 units. Given that the SSS can range from 1 (no symptoms) to 5 (the most severe symptoms) this is quite a large change but quite small changes in SSS -0.2 units or so, can be detected in large studies with statistical significance. These numbers have no intuitively obvious meaning for patients and one can therefore ask – how much of a change in SSS is likely to be noticed by the individual patient? Further once we have a change which is likely to be detectable by the average patient we can go on to ask – does the average patient think this change is ‘worthwhile’? Obviously this second question will be influenced by what the patient had to go through to achieve this degree of change. Issues like this have been studied and are generally considered under the heading of Minimum Clinically Important Difference (MCID) for a given scale.
Global health measures
What if we wish to compare the health benefits of a carpal tunnel operation with that of having a cataract removed, or a hip replaced? For this we need a measure which is not focused on upper limb symptoms. The best known measures of health related quality of life are:
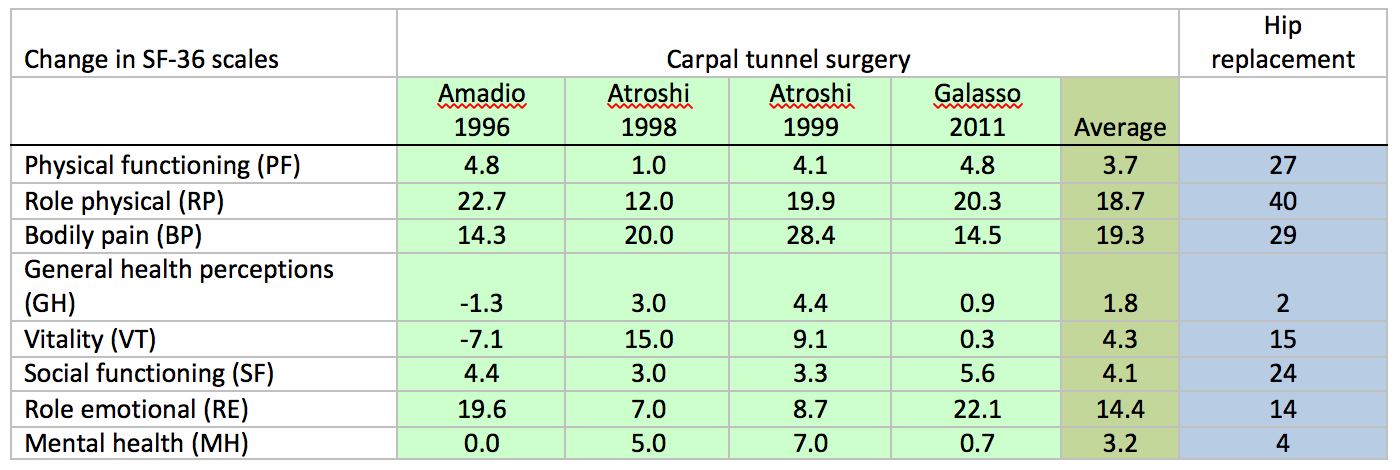
SF-36 – SF6D – the SF 36 is a questionnaire which asks about health issues and bodily functioning in 8 separate domains. In it’s original form it yields 8 separate answers with no recommended way of summing them into an overall score. However a subsidiary measure known as the SF-6D health utility index was proposed later which takes 11 items from the SF-36 questionnaire and combines those to provide a single measure of ‘health’. Several studies have looked at the effect of carpal tunnel surgery on the SF-36:
For CTS we also have some figures for the change in all of these scales 3 months after surgery in the same group of patients (Atroshi 2007)
SF-36 bodily pain scale (range 0-100, higher numbers are better) = +23
SF-6D (range 0.296 to 1, higher numbers are better) = +0.08
SSS (range 1-5, higher numbers worse) = -1.4
FSS (range 1-5, higher numbers worse) = -0.8
The absolute change in these scales can be mathematically transformed into an ‘effect size’ which allows for differences in the starting values in different study populations. Effect sizes of 0.20-0.49 are considered “small”, 0.50-0.79 “moderate” and >=0.8 as “large” (Cohen 1988 Statistical power analysis for the behavioural sciences 2nd Ed Hillsdale, Lawrence Erlbaum Associates Inc.147-51). Using this we can, for example compare the overall effect of carpal tunnel surgery on health, effect size 0.59 for the SF-6D with that of hip-replacement, effect size 1.06 (Feeny 2004)
EQ-5D – simpler than the SF-36 this asks only 5 questions, each of which has 5 options ranging from best to worst. The Euro-QOL working group also suggests the use of a single visual analogue scale question asking subjects to rate their health on a line ranging from “the best health you can imagine” to “the worst health you can imagine”
Finally it is worth observing that ‘health’ is not the only determinant of quality of life and, predictably, there is a questionnaire which attempt to assess overall quality of life, proposed by the World Health Organisation, which has been validated in a variety of different states, racial, social and economic groups - WHOQOL (This link is to a page in the WHO website about substance abuse. I have not been able to find a generic home for WHOQOL on the web as yet).
Revision date - 2nd May 2016